By: Richard M. Blau, chair of GrayRobinson's alcohol beverage, food law, and cannabis industry departments
Now that the federal government has legalized hemp and defined lawful cannabidiol (CBD) produced from hemp, the market for CBD products has moved forward with exponential growth. In 2018, approximately $620 million worth of CBD products were sold in the United States. Popular economic advice platforms such as The Motley Fool report projections from economists and industry experts estimating future growth at approximately $24 billion by 2023. Growing CBD revenue from $620 million in 2018 to $23.7 billion by 2023 delivers a compound annual growth rate (CAGR) of 107%.
While such statistics reflect a maturing market, so, too , do the arrival of product recalls. Recently, several CBD companies announced voluntary recalls of their products.
Summit Labs’ Kore Organic Watermelon CBD Oil
On May 12, 2020, Florida-based Summitt Labs, which produces a wide range of hemp-derived cannabidiol (CBD) products, announced a voluntary nationwide recall of its Kore Organic watermelon tincture after the Florida Department of Agriculture and Consumer Affairs conducted a test on a random sample and found high levels of lead. When ingested, lead can cause various symptoms such as pain, nausea and kidney damage; in prolonged exposure situations, lead poisoning has been shown to contribute to degraded brain functions.
Summit Labs conducted its own test through an accredited, independent lab that found the lead levels in an acceptable range under state law. But, because the Florida officials found excess lead levels in the sample they tested, Summitt quickly moved to withdraw the product from retailers, who have been notified by phone and email.
Any consumer with Lot #K018 Batch #730 in his/her possession is urged to contact Summitt Labs by phone at (833) 810-5673 Monday-Friday 8a.m. to 5p.m. EST, or through the website at www.Koreorganic.com. Any consumer with Lot #K018 Batch #730 also should return this product to the place of purchase for a full monetary refund. If that is denied, the consumer should contact Summitt Labs at the above number for refund information and any other information regarding this recall.
All consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Biota Biosciences’ Cannabidiol (CBD) Complex, Curcumin Complex, and Cannabidiol + Curcumin Injectables
On May 20, 2020, Biota Biosciences voluntarily announced that the company was recalling the following lots in the table below of Cannabidiol (CBD) Complex, Curcumin Complex, and Cannabidiol + Curcumin Injectables to the customer level:
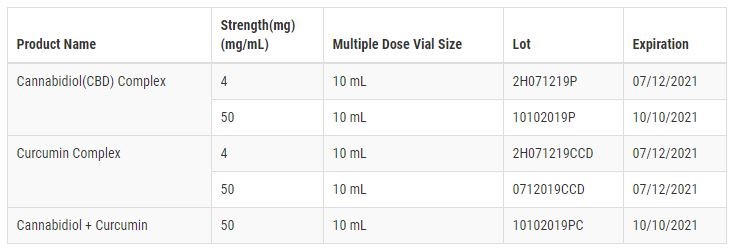
These injectable products are being recalled because they were marketed without FDA approval. The products are deemed by the federal agency to be "unapproved new drugs." Further, the FDA contends that the products are misbranded because the labeling fails to bear adequate directions for use.
Biota Biosciences made the decision to recall after receiving a warning letter from FDA in April advising that the company was violating federal statutes by (i) engaging in interstate commerce of an unapproved new drug, and (ii) failing to properly label the products by neglecting to include directions for use. The company is notifying its distributors and customers by email and is arranging for return of all recalled products. Practitioners or consumers that have product which is being recalled should stop using product and return to place of purchase.
Biota Beta markets its line of CBD vials as pain relievers that serve as an alternative to opioids and can help with detoxification. To date, Biota Biosciences has not received any reports of adverse events related to its recall. However, as part of the recall, the company agreed to include as part of its announcement a warning to consumers that: "Injectable drug products can pose a serious risk of harm to users because they are delivered directly into the bloodstream and bypass many of the body’s natural defenses against toxic ingredients, toxins, or dangerous organisms that can lead to serious and life-threatening conditions such as septicemia or sepsis."
Consumers with questions regarding this recall can contact Biota Biosciences by phone number at (866) 996-2293 Monday to Friday 8:00 am to 4:00 pm PST or by e-mail at hq@biotacbd.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Bonsai Cultivation Recalls Cannabis and Cannabis-Derived Products for Mold
In October of 2019, Bonsai Cultivation, one of Colorado's largest commercial cannabis providers, voluntarily recalled cannabis and cannabis-derived products after plant samples failed mold testing. During a Denver Department of Public Health and Environment (DDPHE) investigation, samples of dried marijuana (including flower, shake, trim, and pre-rolls) were evaluated and contained potentially unsafe levels of yeast/mold.
As a result, all retail marijuana plant material products (including flower, shake, trim, and pre-rolls) bearing the OPC code 403R-00228 sold widely throughout Colorado are subject to the recall:
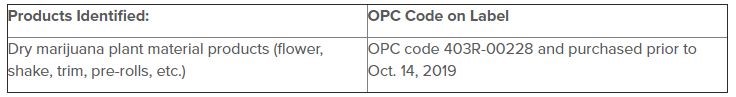
Prior to October 14, 2019, 144 retail stores across Colorado, as well as retail cultivators and retail marijuana infused-product manufacturers received marijuana plant material from Bonsai Cultivation. DDPHE opened an investigation after identifying multiple samples of marijuana plant material had failed total yeast and mold sampling from multiple retail store locations.
DDPHE advised that short- and long-term health impacts resulting from inhalation exposure to mold/yeast may exist depending on the specific product, duration, frequency, and level of exposure.
Preparing for the Next Cannabis/CBD Recall
As the CBD and broader cannabis markets grow under liberalized legalization, the likelihood of more recalls is strong. To avoid a product withdrawal, manufacturers and retailers would be smart to plan ahead, and become familiar with the components of a typical consumer product recall.
Preparation is the first step to competent product recall or voluntary withdrawal management. Key considerations in planning for potential product recalls or voluntary withdrawals from the market should include the following:
- Conduct a complete risk assessment before it’s required. Identify the federal and state public health, agricultural and business risks associated with the business, as well as the key regulatory agencies that oversee the business. Evaluate each identified risk based on the likelihood it could occur, and the impact that would have on the business.
- Follow the risk assessment with scenario-specific crisis planning. Determine, with professional guidance, what consequences would develop if each identified risk incident or crisis occurred. Considerations should include impacts to: (i) production, distribution and retail relations; (ii) employee health and safety; (iii) public reputation and related media coverage; (iv) compliance and licensure consequences; and (v) the economics of necessary remedial efforts.
- Identify recall professionals who can offer expert support. Product safety and quality issues, including recalls, are some of the most challenging situations any company faces. There are many factors to consider, including the expectations of regulatory agencies and consumers. Identifying in advance a competent resource to help implement a competent product recall or voluntary withdrawal allows for a careful, unhurried evaluation process, and will leave the company protected for the future.
As the US Consumer Product Safety Commission advises:
[O]ne of the best ways to ensure that a product recall is effective is to have a recall plan already in place and to execute the plan as quickly as possible. A well-thought out, well-executed recall plan can save lives and prevent injuries in addition to limiting damage to your company’s brand and bottom line.
Long before a recall becomes necessary, cannabis and CBD companies should be fostering an organization-wide recognition of the need for recall readiness. Ensuring that employees understand the link between recalls and consumer safety and satisfaction, as well as the effect well-run recalls can have on corporate success, can help avoid the consequences suffered by the examples outlined above.
Download/print the full article here.
Richard M. Blau is a shareholder with the law firm of GrayRobinson, P.A. He is chair of the law firm's Regulated Products Law Department and heads up the firm’s cannabis industry department focusing on the rules and regulations that govern the production, processing, distribution, sale and dispensing of medical marijuana and related cannabis products.
Mr. Blau has been rated "Band 1" by Chambers USA since 2007. He also is listed in Best Lawyers in America, and is "AV" rated by Martindale-Hubbell. A substantial portion of Mr. Blau’s professional efforts are focused on trade regulations, litigation and dispute resolution involving regulated products such as alcohol beverages, tobacco, firearms and explosives, and most recently medical marijuana and cannabis. He has been involved extensively with the legalization of cannabis in Florida since its outset with passage of the Compassionate Medical Cannabis Act of 2014 (SB 1030) into law on June 6, 2014.
