June, 11, 2018
By: Richard M. Blau, Chair, Food Law Industry Team
The U.S. Food and Drug Administration (FDA) has announced a major recall of pre-cut melons produced by Caito Foods from April 17 to June 7, 2018, due to a risk that the fruits contain Salmonella.
The Salmonella outbreak involves pre-cut melon slices –
- watermelon,
- honeydew,
- cantaloupe and
- fresh-cut mixed fruit containing any one of these melons.
The states affected by this recall are Georgia, Illinois, Indiana, Kentucky, Michigan, Missouri, North Carolina and Ohio.
The retail stores selling the pre-cut melon (with brand name if applicable) include:
- Costco (Garden Highway);
- Jay C;
- Kroger (generic label distributed by Renaissance Food Group);
- Owen’s; Payless;
- Sprouts (Sprouts Farmers Market);
- Trader Joe's (Trader Joe's);
- Walgreens (Delish);
- Walmart (Freshness Guaranteed);
- Whole Foods/Amazon (Whole Foods Market).
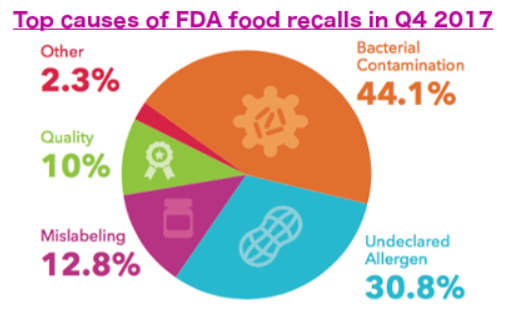
This latest recall caps a five-year spike in food recalls. Food products recalled by the FDA increased 92.7 percent between 2012 and 2017, and recalled pounds regulated by the U.S. Department of Agriculture, which largely oversees meat production, jumped 83.4 percent in the same period. Bacterial contamination, such as Salmonella, E. coli and Listeria monocytogenes, was the most consistent cause necessitating recalls.
About 28 percent of FDA food recalls were for bacterial contamination in 2012. By the end of 2017, that number had grown to 31.3 percent. Undeclared allergens were the top cause of recalled pounds of food by the USDA in 2012 at 35.4 percent; that number increased to 41.2 percent during the past five years.
The cause of this recent recall is Salmonella. Use or consumption of products contaminated with Salmonella may result in serious illness. It can also produce serious and sometimes fatal infections in young children, frail or elderly people and others with weakened immune systems. Healthy individuals infected with Salmonella can experience fever, diarrhea, nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The products were packaged in clear, plastic clamshell containers. Caito Foods has ceased producing and distributing these products as the company and FDA continue their investigation. HOWEVER, because it is possible that products shipped between April 17 and June 7, 2018 still could be on store shelves, consumers should be careful.
The potential that these products are contaminated with Salmonella was discovered through analyzing reports made by state departments of public health. As of June 10, 2018, the number of reported illnesses per state stood as follows: Michigan (32), Indiana (11), Missouri (10), Illinois (six) and Ohio (one). The US Centers for Disease Control (CDC) reports 60 people were infected by the tainted fruits, 31 of which have been hospitalized.
Most persons infected with Salmonella develop diarrhea, fever, and abdominal cramps 12 to 72 hours after infection. The illness usually lasts four to seven days, and most persons recover without treatment. However, in some persons, diarrhea may be so severe that the patient needs to be hospitalized.
Consumers seeking information may call 844-467-7278 Monday through Friday, 7 a.m. to 11 p.m. EDT and Saturday and Sunday, 7 a.m. to 7 p.m. EDT.
Retailers and wholesale customers should check their inventories and shelves to confirm that none of the products are present or available for purchase by consumers or in warehouse inventories. Please contact 844-467-7278 Monday through Friday, 6 a.m. to 10 p.m. CT and Saturday and Sunday, 6 a.m. to 6 p.m. CT to arrange for disposal or return of the product.
For a list of all recalled products from Caito Foods, LLC, please visit the U.S. Food and Drug Administration website.
To read the full article released by the FDA, please visit their website.
